Therapeutics R&D Advanced cell / gene-based biopharmaceutical development and CDMO

-
Duchenne Muscular Dystrophy, DMD
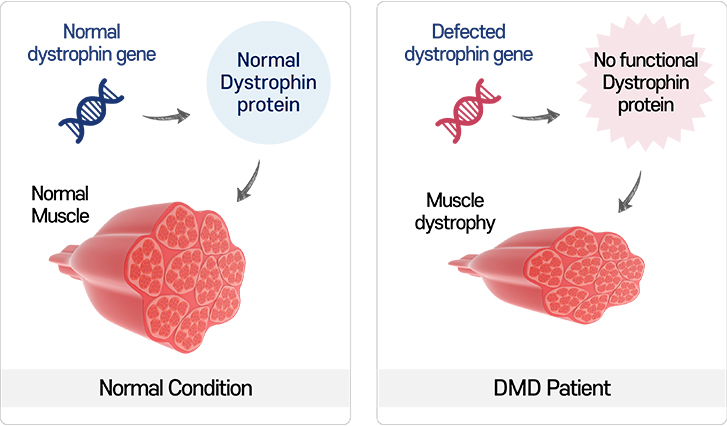
Duchenne Muscular Dystrophy, also known as DMD, is a genetic disease caused by a mutation in dystrophin gene that disables the synthesis of the functional protein.
-
Disease Progression in DMD
Diagnosed from symptoms such as unusual walking gait or frequent loss of balance at the age of 3 to 4. Muscle strength weakens over time as the patients age, making their walk difficult. Respiratory or cardiac functions also progressively weakens over time.

-
The causes of DMD
The protein called dystrophin is an essential molecule supporting the muscle cell membrane. Without the dystrophin, the muscle cells become damaged and the amount and strength of muscle deteriorate.
The major symptoms suffered by the DMD patients are as follow.
-
Major clinical symptoms
-
 Unstable gait.
Unstable gait. Frequent loss of balance.
Frequent loss of balance. Large calf muscle.
Large calf muscle.
-
 Difficulty when rising up, needing to put weight on the knee to rise up.
Difficulty when rising up, needing to put weight on the knee to rise up. Walking on the toes.
Walking on the toes. Difficulty when running or climbing up the stairs.
Difficulty when running or climbing up the stairs.
-
-
Major Biological symptoms

-
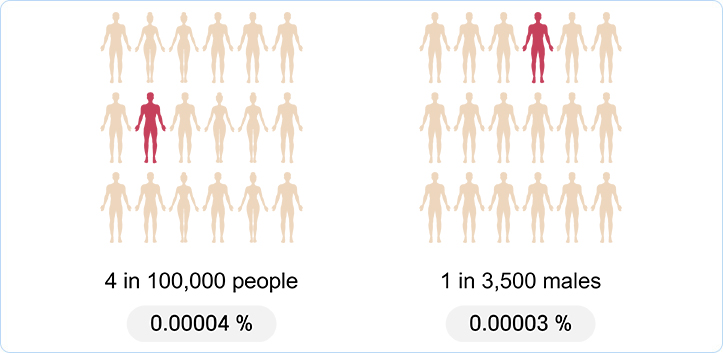
Incidence rate of DMD
-
Treatments
Currently available treatment options for DMD are as follow. Physical therapy to help with mobility
Physical therapy to help with mobility Corticosteroid to slow down the progression of muscle weakening
Corticosteroid to slow down the progression of muscle weakening Treatment for osteoporosis and scoliosis
Treatment for osteoporosis and scoliosis Respiratory support treatment etc
Respiratory support treatment etc
Recently, there have also been several attempts with clinical trials on gene therapy using gene editing.

-
EN001 is a human Wharton's jelly-derived
mesenchymal stem cells (WJ-MSCs)
based cell therapy developed by
ENCell Co., Ltd. for DMD patients.
-
EN001 is a human Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) based cell therapy
EN001 is mesenchymal stem cells derived from wharton's jelly (umbilical cord) using proprietary technology developed by
ENCell Co., Ltd.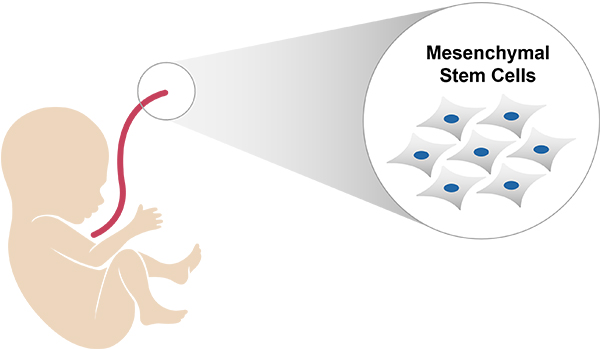
-
Mesenchymal Stem Cells
Mesenchymal stem cell is a type of adult stem cell derived from tissues such as umbilical cord, bone marrow and adipose tissue.
Mesenchymal stem cells are already used as treatments for myocardial infarction, osteoarthritis, amyotrophic lateral sclerosis etc.
There are in total 8 types of mesenchymal stem cell based therapies in the market and they have shown high safety profile.
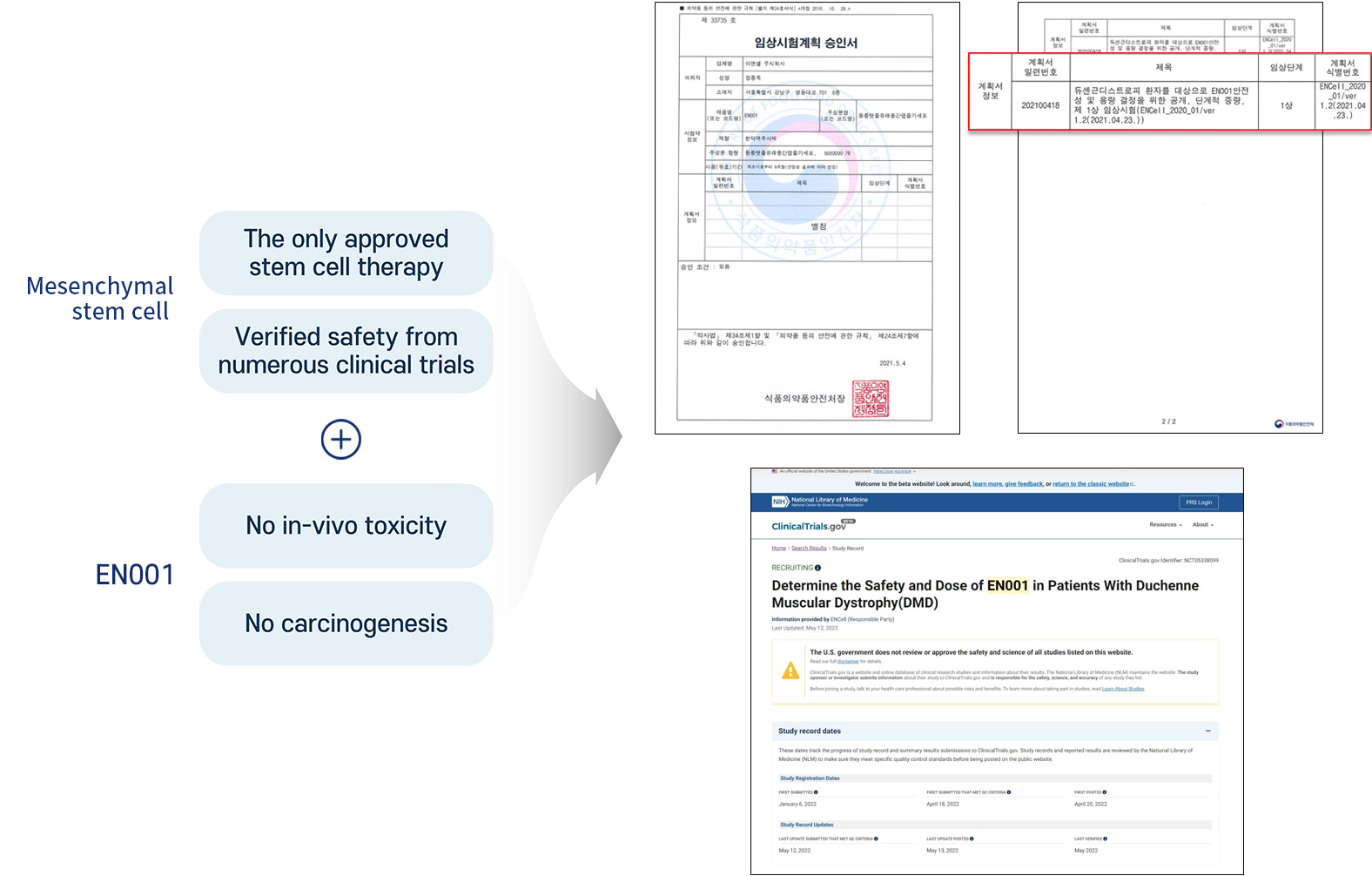
Safety of EN001
Therapeutic efficacy of EN001 in DMD

Therapeutic efficacy of EN001 was confirmed in a preclinical trial using mdx mouse, in vivo model for DMD.
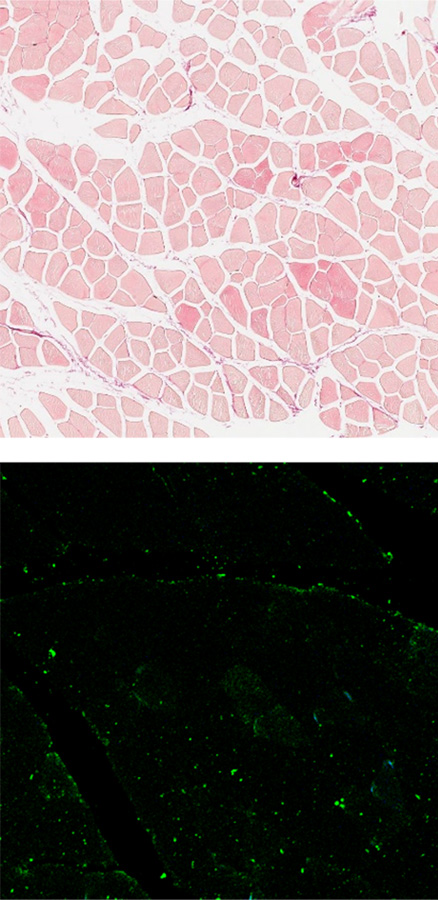
Normal mouse
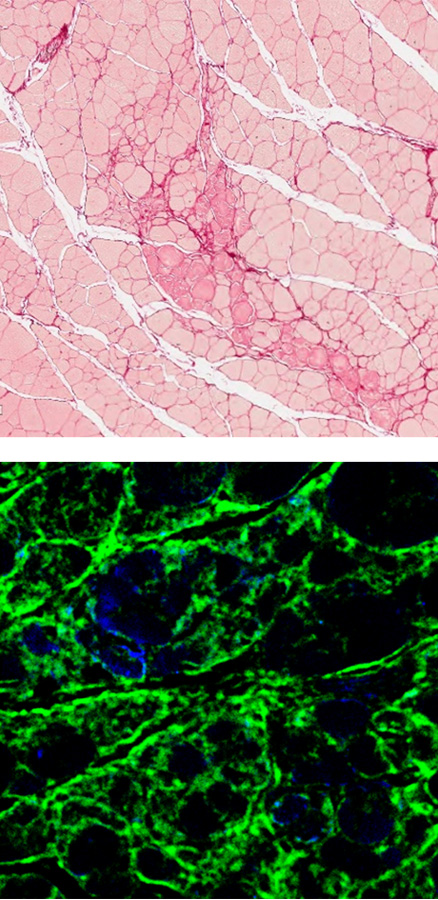
DMD mouse
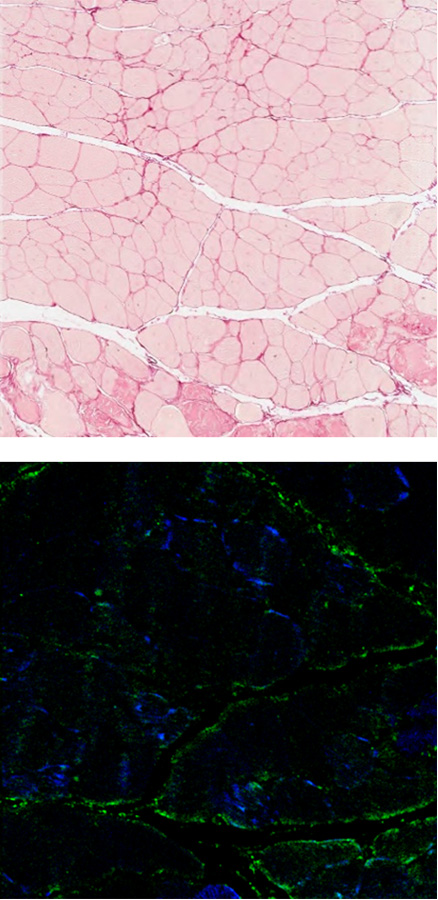
EN001 Administration
The fibrotic muscle tissues are stained with red (top, sirius red staining), and green (bottom, anti-fibronectin). It was observed that there is more fibrosis occurring in gastrocnemius of the DMD mouse compared compared to the normal mouse.
When EN001 was injected into the DMD mouse, significant reduction of the fibrosis was observed.
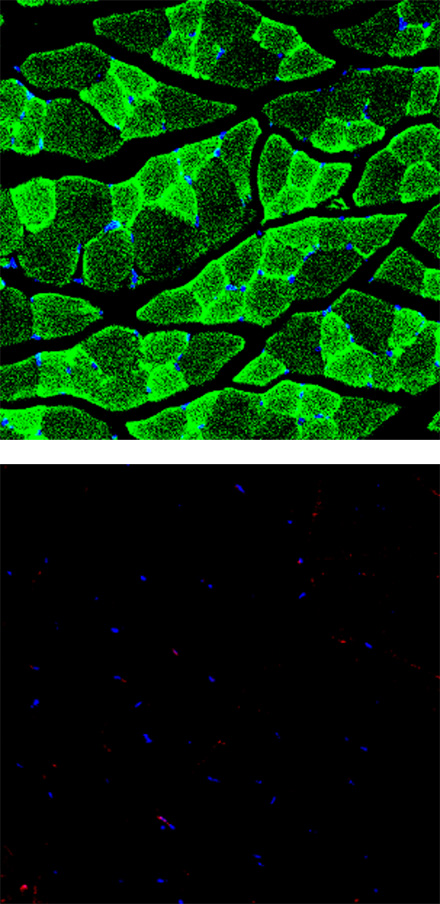
Normal mouse
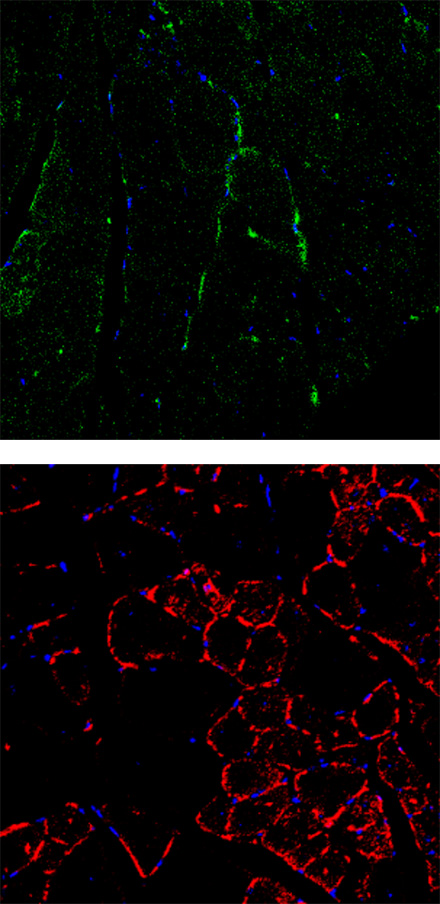
DMD mouse
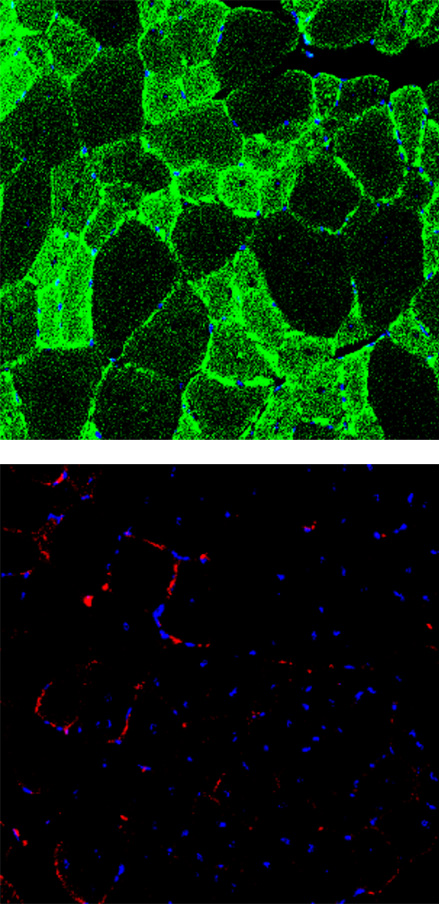
EN001 Administration
Muscle cells are stained with green (top, anti-Myosin Heavy Chain) and the apoptotic cells are stained with red (bottom, anti-Annexin V). It was observed that there are less normal muscle cells and more apoptotic cells in the DMD mouse compared to the normal mouse.
However, when EN001 was administered to the DMD mouse, an increase in the normal muscle cells (muscle regeneration) and a decrease in the apoptotic cells (anti-apoptosis) were confirmed.






