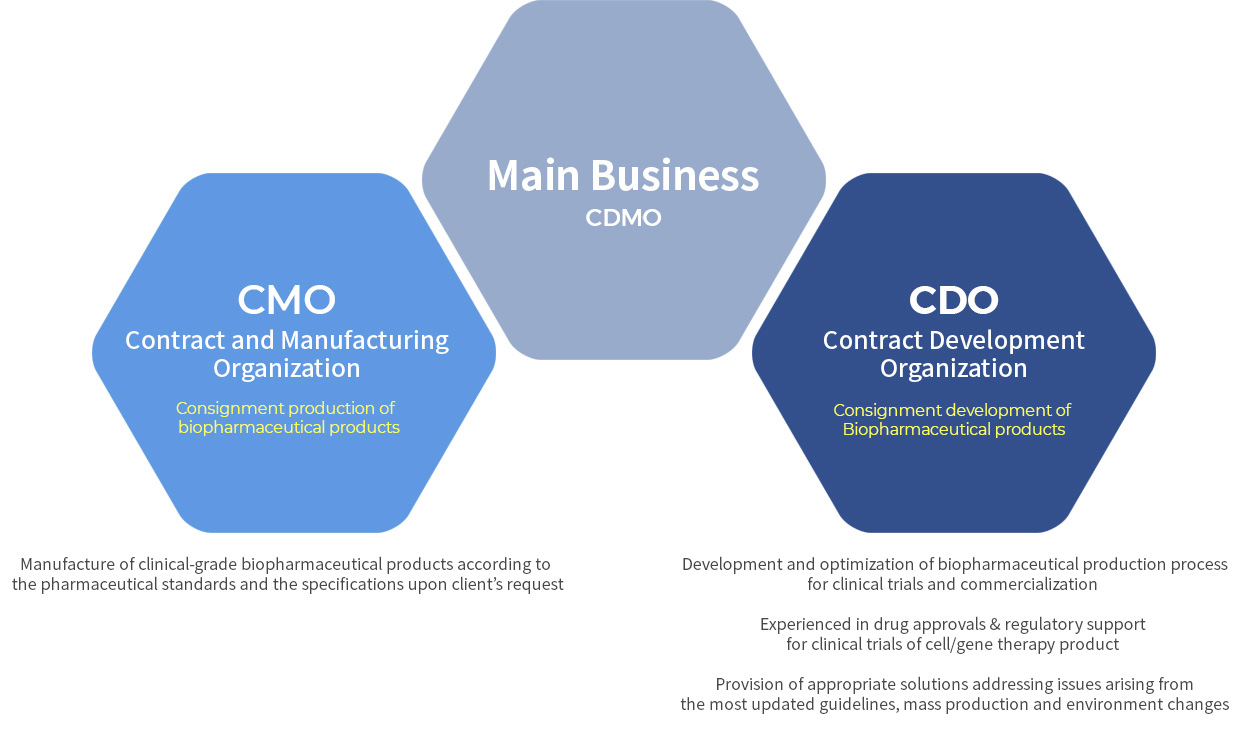
CDMO BUSINESS Advanced cell / gene-based biopharmaceutical development and CDMO
ENCell Co., Ltd. established and maintains premier GMP facilities meeting up to
global standards for cell/gene therapy products.
We provide customized pharmaceutical development services,
from early development stage to approval stage.
CMOConsignment production of leading biopharmaceutical products
- Production of biopharmaceutical product for (pre)clinical trials in compliance with KGMP upon client’s requests
- Leading biopharmaceutical product manufacturing : Multiple source-derived stem cells (Placenta, Wharton’s jelly, Adipose tissue and Bone marrow etc.), CRISPR-stem cell, Exosome, AAV
- Aseptic Process Validation
- Cell characterization: Cell growth, Differentiation potency, Anti-inflammatory effect, Immunogenicity, Cell phenotype etc.
- Essential testing including sterility testing for cell/gene therapy
- Safety testing ( long-term storage)
CDOConsignment development of leading biopharmaceutical products
- Consultations on development of manufacturing processes, quality testing for (pre)clinical trials of biopharmaceutical products
- Specification of the cell culture and manufacturing methods
- Establishing large-scale culture methods according to GMP standards
- Consultations on IND approval
- Establishing test standards for incoming raw materials for production
- Setting up donor tissue suitability test and IPC criteria standardization
- Quality control criteria establishment
- Procure stable packaging and long-term storage system
- Service Scope
- We provide services according to the product development stage upon client’s request
Service Process Flow
-

STEP.01Client request
-

STEP.02NDA agreement
-

STEP.03Consulting
service scope -

STEP.04Cost estimation
-

STEP.05Contract agreement
-

STEP.06Pilot production
-

STEP.07Consignment production and R&D
-

STEP.08Shipment