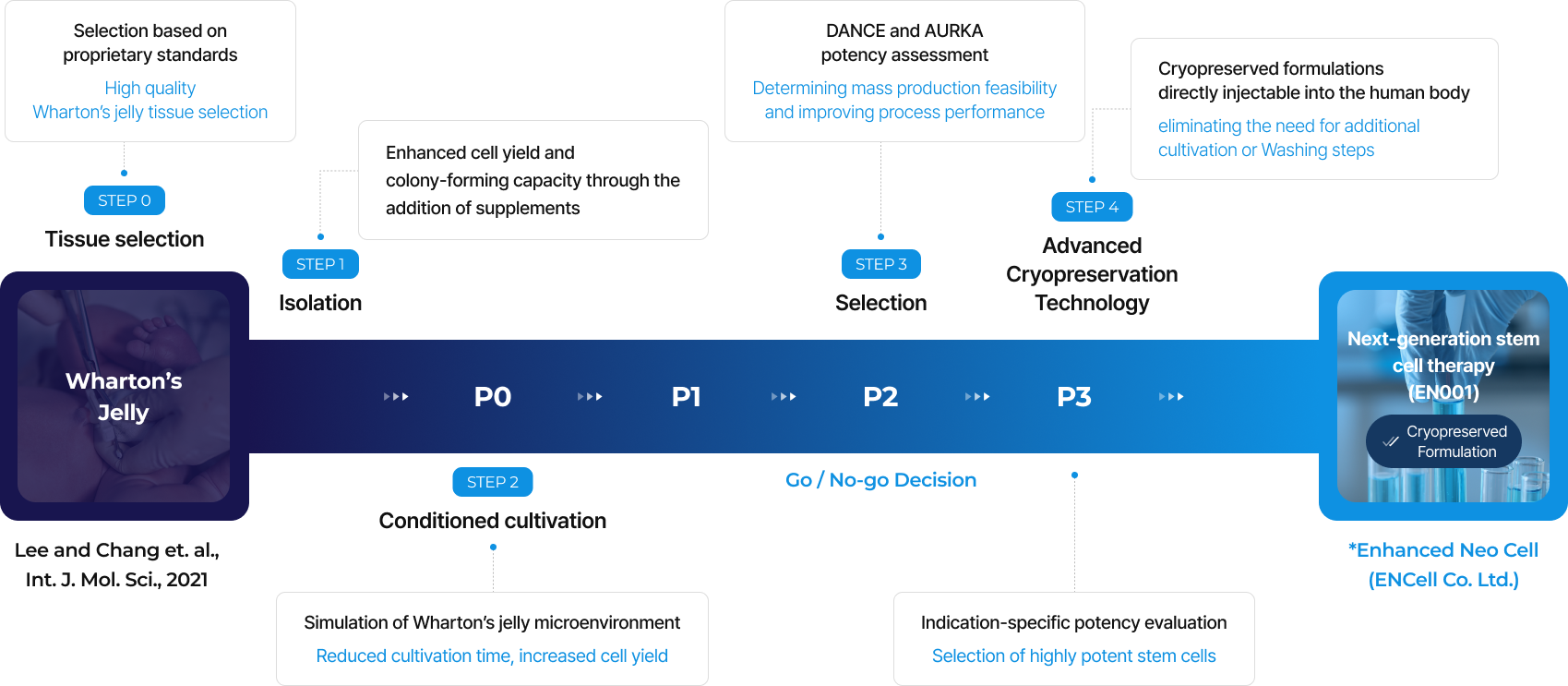
Pipeline
EN001
EN001 is an allogeneic Wharton's jelly-derived
mesenchymal stem cell therapy developed
by ENCell Co. Ltd. for the treatment of Charcot-Marie-Tooth disease.
Next-generation stem cell therapy
- Conventional Cultivation Technology
-
- As the passage number (P) increases, the cultivation period for the cells also lengthens.
- As the cultivation period increases, the cells undergo a process of senescence.
- A decline in stem cell potency resulting from the process of senescence leads to a reduction in the efficacy of the resulting therapy.
- In the end, the result is cells with low therapeutic efficacy and only the required number of total cells.
40-day cultivation
> 1x10⁹ Cells
Minimum required cell count for clinical standards
+α-day cultivation
- EN001 Cultivation Technology
-
20-day cultivation
> 1x10⁹ Cells
Compared to conventional cultivation methods: Higher cell yield & Reduced cultivation period
- DS: Drug substance
- DP: Drug product
ENCell Co. Ltd. has developed a proprietary technology that allows for the isolation of EN001 stem cell therapy from Wharton's Jelly.
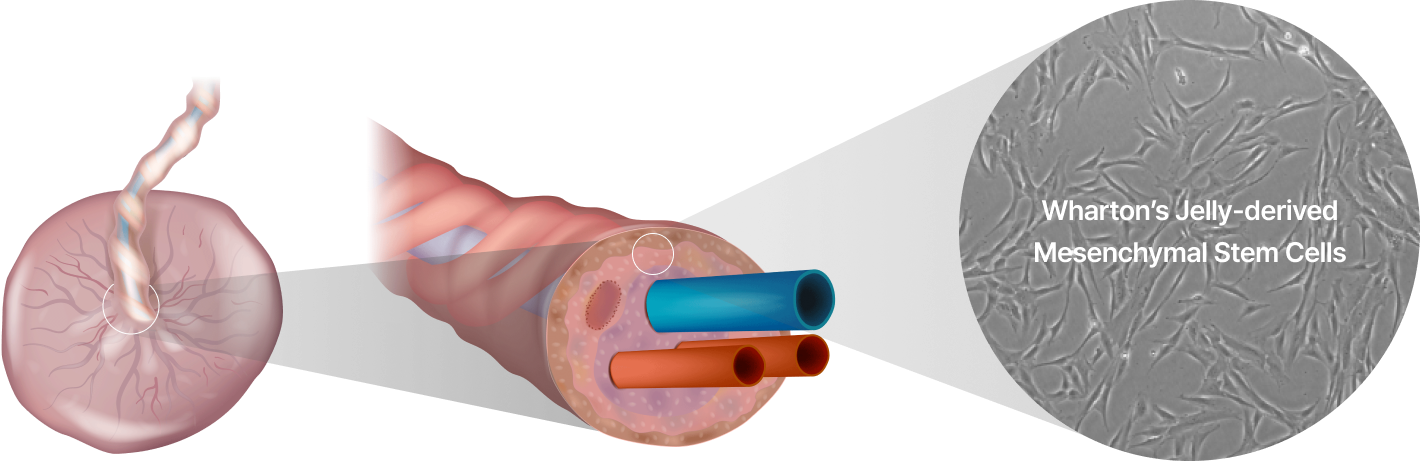
- Mesenchymal Stem Cells (MSCs)
-
- Mesenchymal stem cells are a type of adult stem cell obtained from tissues such as the umbilical cord, bone marrow, and adipose tissue.
- These cells are employed in the treatment of a number of conditions, including myocardial infarction, osteoarthritis, Amyotrophic lateral sclerosis and Crohn's disease.
- In domestic and global regions, eight types of mesenchymal stem cells have been developed into drugs and are currently on the market, with a proven track record of safety.
Safety of EN001
EN001 is a therapy that utilizes mesenchymal stem cells, identical to the eight - stem cell-based therapies currently sold worldwide.
Over 8,000 clinical trials conducted globally have confirmed the safety of mesenchymal stem cell therapies, with no severe side effects reported.
EN001, developed by ENCell Co. Ltd., underwent rigorous safety verification through certified testing institutions (KIT) and was approved for clinical trials by the Ministry of Food and Drug Safety (May 4, 2021) after a thorough review process.
- Mesenchymal Stem Cells (MSCs)
- The Only Approved Stem Cell Therapy
- Track Record of Safety Confirmed Through Numerous Clinical Trials
- EN001
- No systemic toxicity
- No tumor formation
Charcot-Marie-Tooth Disease
- Charcot-Marie-Tooth disease, CMT
- CMT is an inherited genetic disorder caused by nerve sheath damage, resulting in motor function and sensory impairments.
Progression of Charcot-Marie-Tooth Disease
The onset of symptoms is dependent on the individual. Over time, the muscles in the feet and hands gradually weaken and atrophy, which can result in sensory loss.
The symptoms can range from a condition that has no impact on daily life to severe cases that require the use of a wheelchair.

Causes of Charcot-Marie-Tooth Disease
To date, approximately 70 causative genes have been identified. However, the causative genes for about 50% of patients remain unknown.
For nerves to function correctly, a substance called myelin (a lipid-based protein) is required.
Charcot-Marie-Tooth disease is a condition that impairs nerve function due to damage to the myelin sheath.
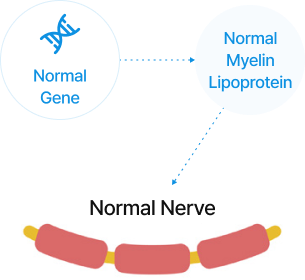
Healthy Individual
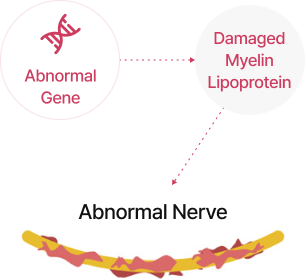
CMT Patient
The primary symptoms experienced by patients diagnosed
with Charcot-Marie-Tooth are as follows
Clinical Symptoms
- Muscle weakness begins in the lower legs.
- Loss of sensation in the feet and hands, including inability to feel pain or temperature.
- Calf muscles shrink (resulting in a "stork leg" appearance).
- Deformities such as high foot arches (elevated instep) and claw toes (Z-shaped curled toes).
Biological Symptoms
Nerve sheath damage
(demyelination)
Loss of muscle cells
Reduced muscle regeneration
Charcot-Marie-Tooth Disease Patient Population
Global: Approximately 1.4 million
Domestic: Estimated around 10,000
Prevalence 1 in 2,500 people (0.00004%)
Treatment Methods
The current treatment methods for Charcot-Marie-Tooth disease are as follows
- The use of assistive devices is recommended to prevent deformities.
- The recommended course of treatment is physical or occupational therapy.
- Medications are also available to slow disease progression, including Ascorbic acid, PTX3003, and others.
Recently, clinical trials have commenced for genetic therapies involving gene correction.
Therapeutic Impact of EN001 on Charcot-Marie-Tooth Disease
Publications on EN001 as a treatment for Charcot-Marie-Tooth (CMT) from ENCell Co. Ltd.
- Nerve Sheath Regeneration Won et. al., STEM CELLS, 2020
-

- Inhibition of Muscle Cell Apoptosis (Anti-apoptotic Effect) Kwon et. al., Mol. Ther., 2016
-

- Schwann cell Proliferation and Myelination Oh et. al., Neurobiol. Dis., 2024
-

EN001 Therapeutic Efficacy Confirmed in Preclinical Studies in the Charcot-Marie-Tooth (CMT) C3 Mouse Model
-

Wild-type mouse
-

CMT mouse model
-

EN001 Treatment
The myelin sheath, which insulates the nerves, is shown in dark gray in the images.
In the CMT model, the myelin sheath is thinner than in the normal model, and its shape appears irregular.
However, when EN001 was administered to the Charcot-Marie-Tooth model, the thickness of the myelin sheath increased, and its shape became healthier.
-

Wild-type mouse
-

CMT mouse model
-

EN001 Treatment
Healthy muscle cells within the muscle tissue are stained green.
Compared to the normal mouse model, the Charcot-Marie-Tooth mouse model shows a decrease in healthy muscle cells.
However, when EN001 was administered to the Charcot-Marie-Tooth mouse model, an increase in healthy muscle cells was observed (muscle regeneration effect).

