Contract Development and Manufacturing (CDMO)
ENCell Co. Ltd.’s CDMO
Multi-product GMP Production Technology-based CDMO Services
ROCKET(RObusting Customer’s Knowledge & IP through ENCell GMP Technology)platform
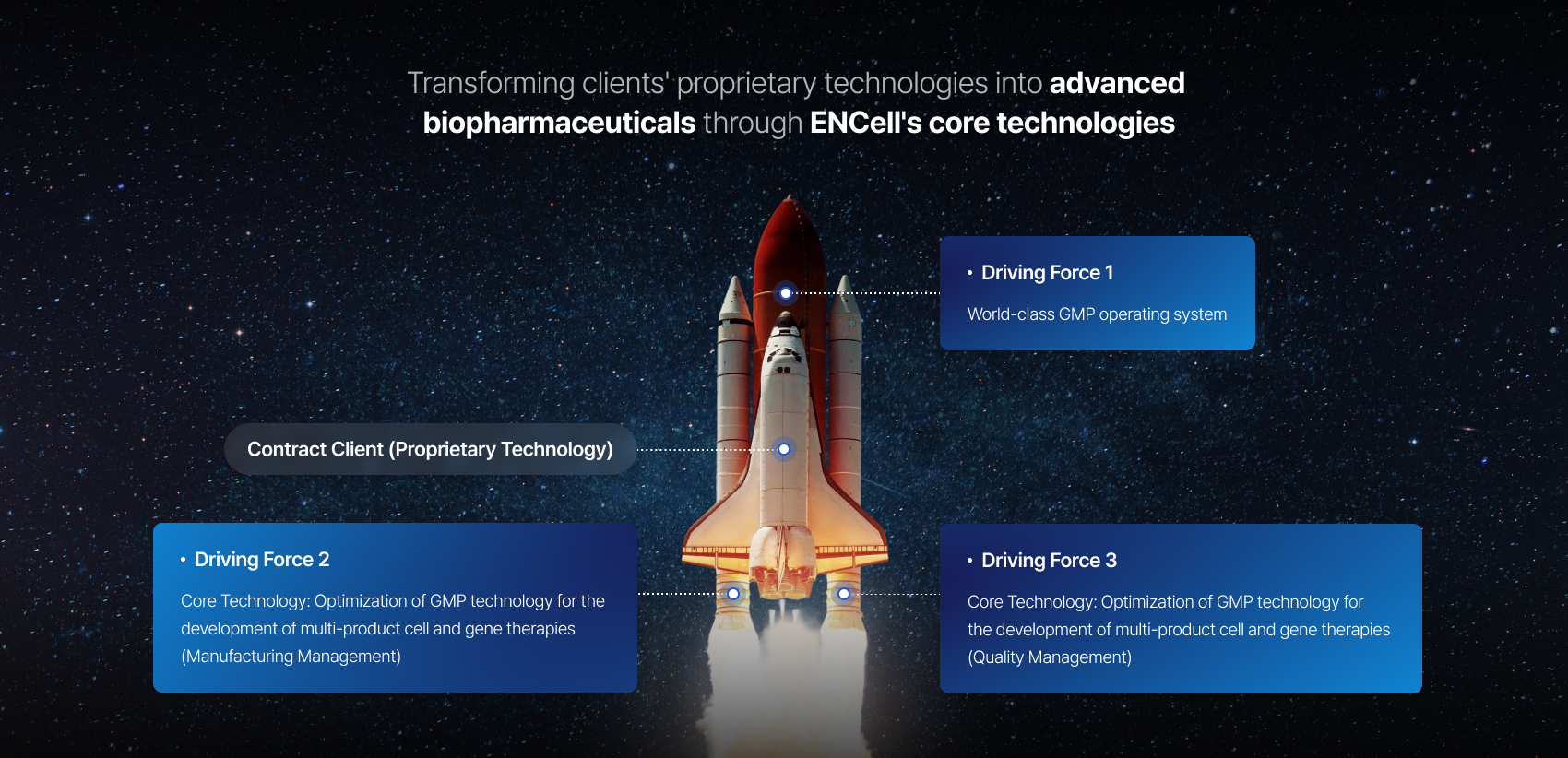
Comprehensive consulting services for every stage from R&D to regulatory approval
- Providing services that meet regulatory requirementsHuman cell management, advanced biopharmaceutical manufacturing and cell processing facilities
- Research & Development
-
Discovery of material candidates
Core manufacturing process development
Proof of Concept (POC) studies (in vitro, in vivo)
- Process Development
-
Process development for clinical trial material manufacturing
New formulation development
Process optimization (QbD-based)
Characterization and Analytical Methods
- GMP manufacturing
and storage -
GMP technology transfer
Validation of manufacturing and assay methods
Cell bank manufacturing and storage (MCB, WCB)
DS/DP manufacturing and storage
- Testing & Verification
-
Safety & Security
Stability testing
Toxicity and biodistribution studies (in vitro, in vivo)
Efficacy testing (in vitro, in vivo)
Logistics Services
- Regulatory support
-
Regulatory engagement support
CMC strategic consulting
IND filing support
Orphan Drug and Fast Track designation support
Technical Research for CDMO Process and Quality Analysis
- End-to-end services for advanced biopharmaceuticals
GMP manufacturing
process development
for cell and gene therapy
GMP quality
analysis development
for cell and gene therapy
Process optimization
and establishment of
key process standards
GMP technology
transfer
Viral targeting & production
platform development
Viral vector
systems development





