Contract Development and Manufacturing (CDMO)
GMP manufacturing and quality control expertise


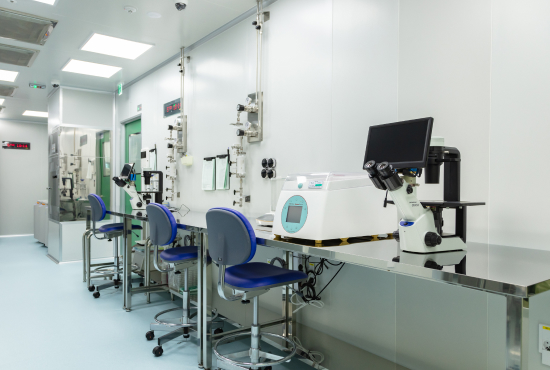
Introduction to GMP Manufacturing Facility
[GMP Facility 1] Cell Therapy
| Location |
Seoul, Gangnam-gu, 
|
Area | 580m² | |
|---|---|---|---|---|
| CAPA | Production | 116 units (based on Cellstack containers) / 73,776㎠ (based on surface area) | ||
| Storage | 110,800 vials (vial-based) / 4,500 bags (bag-based) | |||
| Structure |
|
|||
| Application | Clinical-grade cell and gene therapies (Naïve / Pre-conditioned / Gene-modified MSC, T cell, NK cell) | |||
[GMP Facility 2] Cell & Gene Therapy
| Location | Hanam, Gyeonggi Province | Area | 3,443㎡ | |
|---|---|---|---|---|
| CAPA | Production | 198EA(based on Cellstack containers) / 125,928㎠ (based on surface area) | ||
| Storage | 260,800 vials (vial-based) / 10,800 bags (bag-based) | |||
| Structure |
|
|||
| Application | Clinical-grade cell therapies (Naïve / Pre-conditioned / Gene-modified MSC, T cell, NK cell) | |||
[Exterior view of GMP facility 1 & 2]
[GMP Facility 3] Gene Therapy
| Location | Hanam, Gyeonggi Province | Area | 1,161 m² | |
|---|---|---|---|---|
| CAPA | Raw Materials | 20 Lots/Year (1x10¹² vg/ml) | ||
| Finished Products | 20 Lots/Year (10,000 vials/year) | |||
| Structure |
|
|||
| Application | Clinical-grade gene therapies (Viral Vectors: Lentivirus, Adeno-associated virus, Adenovirus) | |||
| Key Features | Airborne Particle Control System, Separate Biopharmaceutical DP Production, Single-Use & Closed Systems | |||