Contract Quality Testing
Tests

Sterility Testing
This is the final procedure for testing finished products for microbial contamination.
It includes testing the suitability of the test method and improving the accuracy and reliability of sterility testing.
- Direct inoculation method
- Membrane filtration method
- Rapid microbial detection method

Endotoxin Testing
Detects endotoxins, a component of lipopolysaccharides found in Gram-negative bacteria.
Checks for endotoxin contamination in cell and gene therapy manufacturing processes or in various culture media used in operations.
- Chromogenic (colorimetric) method

Mycoplasma Contamination Testing
Identifies the presence of Mycoplasma bacteria that may be contaminated by animal raw materials, media, or manufacturing environments used in the cell culture process.Includes testing for activation and growth conditions of mycoplasma strains via the Mycoplasma Culture Inhibition Activation Test.
- Direct culture method
- Rapid nucleic acid amplification (PCR) method
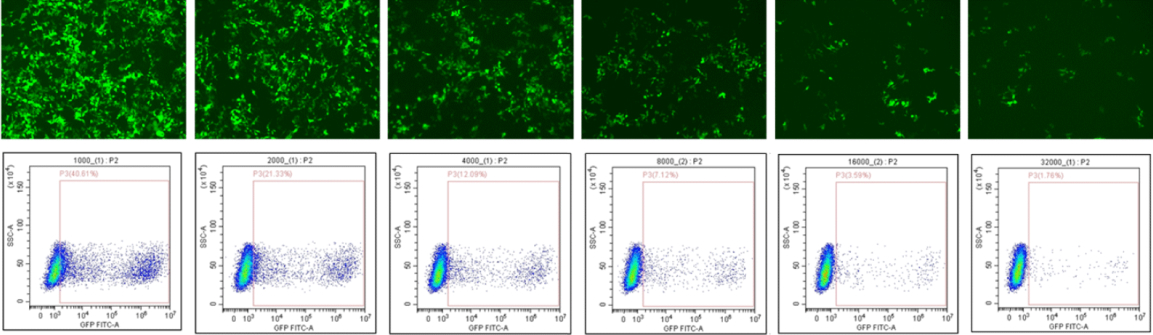
In-vitro adventitious virus testing
Determines whether adventitious viruses introduced from animal-derived substances, such as raw media used during the cell and gene therapy manufacturing process, have contaminated the final product (Performed as a 28-day cell culture test)
- Cytopathic effect (CPE) assay
- Hemagglutination Assay
- Hemadsorption Assay
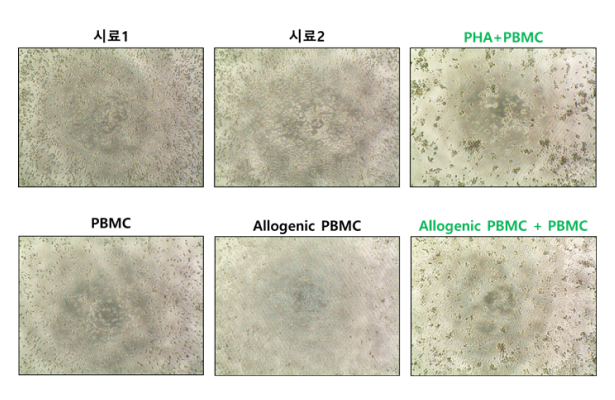
Mixed Lymphocyte Reaction (MLR) Assay
Evaluates the immunologic compatibility and safety of cell therapy products using a T-cell activation model involving interaction between donor and recipient materials.